INFO 10-1997: The gemstone connection: ISO links comets to stars and Earth's origin
28 March 1997
Comets contain the remnants of the raw materials that built the Earth and the other planets of the Solar System. Emphatic confirmation of this long-standing belief of astronomers comes from the detection of the mineral olivine in Comet Hale-Bopp, by ESA's Infrared Space Observatory, ISO. The 28 March issue of the US journal Science carries a report on this result by a European and American team led by Jacques Crovisier of l'Observatoire de Paris-Meudon."ISO sees the same materials in Comet Hale-Bopp as in dust clouds around other stars," Crovisier comments. "A key ingredient of both stardust and comet dust is olivine in crystalline form. This is also one of the main constituents of the Earth's interior. Now we can say with real confidence that we stand on a congealed pile of mineral dust, like that contained in the comets swarming around the Sun 4500 million years ago."
Olivine predominates in the mantle below the Earth's thin crust, and crops up at the surface as the olive-coloured gemstone, peridot. Geologists also value minerals rich in olivine, as important sources of chromium, platinum and diamonds.
Ingredients of Comet Hale-Bopp's vapour and dust emit characteristic infrared wavelengths, many of which are clearly observable only in space. The team took advantage of ISO's unparalleled ability to analyse intensities across a wide band, from 2 to 200 microns wavelength, using three of the spacecraft's instruments: the Short-Wavelength Spectrometer SWS, a short-wave spectrometer within the photometer ISOPHOT, and the Long-Wavelength Spectrometer LWS.
 |
|
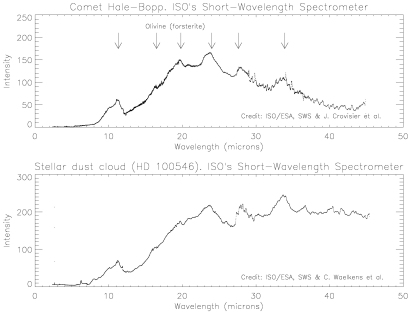
Infrared emissions from Comet Hale-Bopp signal the predominance of a crystalline form of olivine, in the mineral dust emerging from the comet. The same material is conspicuous in a dust cloud around a young star, where planet formation may be in progress. |
ISO's SWS reveals the olivine dust in the great comet in a distinctive cluster of emission peaks (11.3, 16.5, 19.8, 24.0, 27.6 and 33.9 microns). These are characteristic of crystalline forsterite, a form of olivine rich in magnesium. The infrared fingerprint is wholly different from that of the pyroxenes, commonplace silicates of the Earth's crust.
Last year, astronomers using the same instrument on ISO reported strong hints of olivine, in emissions around 33 microns from dust clouds surrounding half a dozen aged and dying stars. This is where Nature makes the olivine from chemical elements released from the old stars. Other SWS users recorded the full cluster of forsterite emissions in a dust cloud surrounding a young star, where planet formation may be in progress. The similarity between the spectra of this star (called HD 100546) and of Comet Hale-Bopp is astonishing. Although the objects are separated by hundreds of light-years, and huge differences of scale, ISO sees the same dominant mineral in both.
Comets carry ices too, in their cargoes - principally frozen water, carbon monoxide and carbon dioxide. ISO has shown them to be the principal ices in interstellar space, where they exist on small grains. In Comet Hale-Bopp, ISO detects and measures these materials as they turn into vapour. Crovisier and his colleagues report in Science the rate at which Comet Hale-Bopp sweats off weight in the heat of the Sun.
On 27 September 1996, when the comet was still 444 million kilometres from the Sun, it was shedding water vapour into space at 10 tonnes per second, carbon monoxide at 11 tonnes per second, and carbon dioxide at 5 tonnes per second. Altogether Comet Hale-Bopp's loss of these materials amounted at that time to 2.2 million tonnes per day. Counting molecules rather than mass, the water vapour, carbon monoxide and carbon dioxide were vaporizing in the ratio 10 to 6 to 2.
The temperature at which the water molecules first formed in space, billions of years ago, was about minus 250 degrees C. Crovisier's team arrive at this answer by distinguishing with SWS the infrared signatures of two kinds of water molecules, at 2.6 to 2.9 microns. When water molecules form at ordinary temperatures, the nuclei of both hydrogen atoms spin the same way, in three cases out of four. At very low temperatures of formation, as in interstellar space, contrary directions of spin become commoner. The best match to the ISO spectrum comes from a ratio of 2.45 to 1 for the two molecular types, corresponding to molecule-making at 25 degrees above the absolute zero of temperature.

